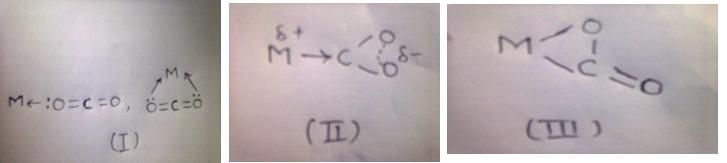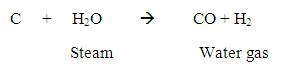Technical paper: Hydrogen Fuel from Carbon dioxide
Author: K Shivaraj Kumar, 2nd year, B Tech, Chemical Engineering
College: Rajiv Gandhi University of Knowledge Technologies, Basar
ABSTRACT:
Worldwide use of Hydrocarbon fuels is majorly health hazarding, and it is daily increasing green house effect and global warming by releasing carbon dioxide, carbon monoxide, oxides of nitrogen, methane, CFCs, etc. The major contribution among those gases is given to carbon dioxide. To combat green house effect, the emission of carbon dioxide must be reduced. This can be done by improving energy efficiency in automobiles, industries, household heating and lighting. Non-fossil fuel sources should be encouraged. The fuel must be high energy efficient and the combustion product of fuel should be environmentally benign. That kind of fuel is HYDROGEN FUEL…This is produced from CARBON DIOXIDE.
INTRODUCTION:
Carbon dioxide is a naturally occuring chemical compound composed of two oxygen atoms each covalently double bonded to a single carbon atom. It is a gas at standard temparature and pressure and exists in Earth`s atmosphere in this state, as a trace gas at a concentration of 0.039 percent by volume. It has four non-bonding lone pair electrons, thus it acts as a ligand. Each oxygen atom carries two non-bonding lone pair electrons, so there are two donor atoms available and those make CO2 act as bi-dentate ligand. The CO2 activated by means of transition metal compounds. The complexes formed from CO2 in reactions with low-valent, high oxidation state and strong lewis acids such as Rhodium, Platinum, Chromium, Iron and Ruthenium.
In metal carbon dioxide complexes, CO2 serves as a ligand, which can felicitate the conversion of CO2 to other chemicals.
CO2 displays several alternate modes of coordination with transition metal compounds via oxygen by donation of the oxygen lone p-electron pair to the vacant orbital of the metal(I), by electron donation from metal to carbon orbital with formation of the metallo-acid derivative(II), or finally by way of π-complex formation via C=O double bond(III).
A priori on the basis of properties of the molecular orbital of CO2 one may expect the formation of the complexes of the first type is favorable with transition metal compounds of the higher oxidation states and strong lewis acids, while the formation of complexes of the second and third types is favorable with transition metal compounds of the lower oxidation states.
FIRST IDEA:
Formation of complexes of the first type;
Here M – Metal should be of higher oxidation state and a strong lewis acid…Ex: Rhodium, Platinum, Chromium, Iron and Ruthenium, etc.
In CO2 is composed of two oxygen atoms each covalently double bonded to a single carbon atom with bond energy is 192.1kcal and bond length is 1.163Å.
When high energy supplied to the complex compound of CO2 of first type, the complex compound absorbs the energy in the form of Vibrational, Rotional, Kinetic, etc. then the compound will vibrate and rotate…As a result, Sigma bond becomes weak and π-bonds become weaker. As the central metal of the complex compound is of higher oxidation state, it will attract Oxygen atoms…so Oxygen moves and stretches towards the metal and finally breakage between Carbon and Oxygen atoms takes place. Now carbon is free from oxygen atoms…metal will form metal oxides. In this reaction, one can easily separate the carbon from solid metal oxides. Finally, carbon is converted from CO2.
REACTIONS:
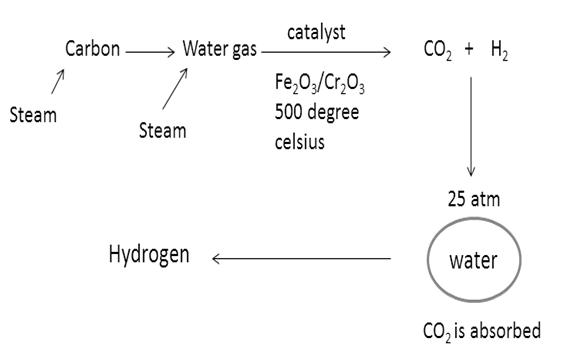
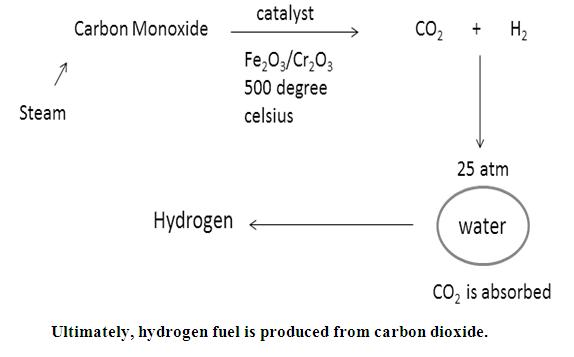
Now, water gas will be produced by passing steam over this carbon gas.
Now, by Bosch’s process, one can convert water gas to HYDROGEN FUEL.
Steps involved are:
Water gas is mixed twice its volume of steam and passed over a mixer of Ferric oxide and Chromium oxide heated to 500℃. The mixture acts a catalyst and helps in the conversion of carbon monoxide to carbon dioxide.
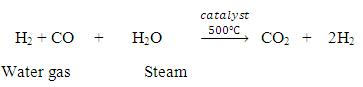
The mixture of CO2 and H2 is passed through water under a pressure of 25 to 30 atmospheres. CO2 completely dissolves in water.
Hydrogen fuel is produced.
SECOND IDEA:
Now, CO2 as a monodentate:
There are certain ligands which have two or more donor atoms but in forming complexes only one donor atom is attached to metal ion. Such ligands are called Ambidentate ligands. Examples of such ligand is:
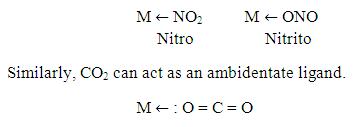
Similarly as done in case of bi-denatate, one can supplies high energy to the complex compound of CO2. The compound absorbs the energy in the form of Vibrational, Rotional, Kinetic, etc. then the compound will vibrate and rotate…As a result, Sigma and π-bonds become weaker between carbon and donor oxygen atom. As the central metal of the complex compound is of higher oxidation state, it will attract donor Oxygen atom only…so donor Oxygen atom moves and stretches towards the metal and finally breakage between Carbon and donor oxygen atoms takes place….and this will result in formation of carbon monoxide which can be later separated from the metal oxide.
Now, by Bosch’s process, one has to pass the steam over Carbon monoxide…this will result in the mixture of carbon dioxide and Hydrogen.
REACTIONS:
The mixture of CO2 and H2 is passed through water under a pressure of 25 to 30 atm. CO2 dissolves completely in water…and this will result in formation of Hydrogen fuel.
ADVANTAGES:
The world’s fossil fuel reserves are being depleted at an alarmingly fast rate. Faced with this dilemma, scientists have made intensive efforts in recent years to develop alternative sources of energy. Hydrogen has been considered as one of the alternate source of energy.
One major advantage of using hydrogen as a fuel is that it is environmentally clean, giving only water as a combustion product, so it is a “clean fuel”. If hydrogen is burned in air, small amount of nitrogen oxides can be produced because of high temperature combination of nitrogen and oxygen, but the combustion products are free from CO, CO2, SO2, unburned hydrocarbons and other environmental pollutants that result from the combustion of petroleum fuels.
The advantage with hydrogen is that the heat of combustion of hydrogen per gram is higher than any other fuel. The energy released by combustion of hydrogen with oxygen is 142 KJ/gr while other fuels give energy below 48 KJ/gr.
Reaction:
CONCLUSION:
High energy efficient fuel is produced from Carbon dioxide besides combating Green house effect and Global warming.
REFERENCES:
1. A Text Book Of Inorganic Chemistry by Dr.O.P.Tandon.
2. Reactions of Carbon dioxide with transition metal compounds by M.E Vol’pin and I.S. Kolomnikov. Institute of Organo-Element Compounds, Vavilova 28, Mascow V-312.
3. www.iupac.org
4. www.alchem.com
5. www.sciendirect.com