Technical Paper Title: OSMOTIC POWER
Authors: ROHINI, 3rd BTech, EEE
College: Bhojreddy engineering College for women
Authors: MD.AHMAD PEER, 4th year BTech, ECE
College: Ayaan College of Engineering & Technology
ABSTRACT
We can’t continue using several of our energy sources we gain energy from today. For example fossil fuels contaminate our environment and we are also running out of them. It is therefore necessary to find other ways of producing energy. This report focuses on one of those alternatives; osmotic energy. Osmosis means passage of water from a region of high water concentration (often freshwater) through a semi permeable membrane to a region of low water concentration (often Na Cl). The membrane only lets water molecules pass. Salt molecules, sand, silt and other contaminants are prevented to do so. Several physiological processes use this osmotic effect. For instance, our body uses it to bring water back from the kidneys, and plants use osmosis to keep the water pressure inside the plant at a fixed level. Since scientists have found a way to build semi permeable membranes, we can use the osmotic effect and convert it to mechanical energy. This paper focus on principle, generation, advantages, economic and environmental prospects of osmotic power and how this technology contributes value to renewable energy production. The scope of this paper covers, the fundamental concepts of osmosis, the real-world implementation of this technology along with its limitations, and finally the future direction in which the osmotic power generation is headed towards.
INTRODUCTION
THE OSMOTIC PROCESS:
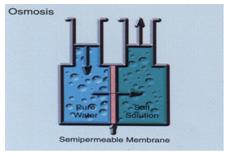
The main thing with osmotic energy is transportation of solutions (often pure water and salt-water), separated by a special filter, a membrane. In the osmotic process it is not possible to use an ordinary filter. You need a “Semi permeable membrane”. A semi permeable membrane is an organic filter with extremely small holes. The membrane will only allow small molecules, like water molecules, to pass through. The thin layers of material cause this and that is what the osmotic energy process is all about.
The picture here on the right shows a simple test rigg for this process. The left side contains pure water. The right side contains a solvent with water and salt (NaCl). The only thing that separates them now is the semi permeable membrane. The process is about to begin.
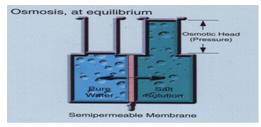
When the process gets started the pure water on the left side aspires to decrease the salt-concentration on the right side of the membrane. The amount of water on the right side will now increase and create an “Osmotic head pressure”. We can use this pressure, for example, to force a water- turbine to rotate. The amount of freshwater that will pass through the membrane depends on the salt-concentration in the salt-water, before the osmotic process begins. For instance, if the salt-concentration from the beginning is 3.5%, the osmotic pressure will be about 28 bars.
The problem with the test rigg is that the salt-concentration in the salt-water will decrease and the process will slow down. The only way to fix this is to continuously, empty and refill both the left and the right side. This must be done very quickly to avoid run-interference. Another problem is that the membrane can, and will wear out because of all silt and other contamination that will get stuck in the membrane. If we don’t consider this fact a membrane’s length of use is about 6 months. This sort of process could not only be used for energy purpose. The main use area today is Reverse Osmosis, where you create a pressure larger than the osmotic head pressure and push the salt water through the membrane. From this process you gain fresh water out of salt-water.
HOW OSMOTIC POWER WORKS When freshwater meets saltwater, for example where a river flows out into the sea, enormous quantities of energy are released. This energy can be utilized to generate power through the natural phenomenon ofosmosis.
Osmotic power plants utilize the osmotic pressure difference between seawater and freshwater to drive a turbine, which in turn generates electricity.
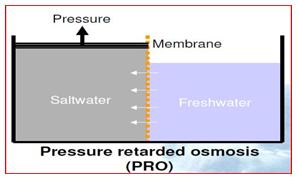
The working of osmotic power can be described as a technology that utilizes the power released when freshwater mixes with saltwater. Hence it is somewhat of a reverse process of desalination. Pressure retarded osmosis, or the PRO concept harnesses the energy in this process to run turbines.
As freshwater passes through a semi-permeable membrane to a saltwater chamber with limited volume, pressure will theoretically rise to a maximum of 26 bars. The operating pressure in a power plant will be in the range of 11 to 15 bars, an equivalent to a water head of 100 to 145 meters in a hydropower plant, generating about 1 MW/m3s freshwater.
Osmotic power plants can be designed for installation in a number of coastal sites. A sea level PRO power plant can be designed as a run- of-the-river hydropower plant, as it takes freshwater in from a river outlet. Another is a combined conventional SHP and membrane plant that is buried in appropriate geological sites near the coastline, utilizing gravity for extra pressure. Many other flexible applications are possible.
Osmotic power is highly efficient and can operate at full capacity for more than 7000 hours a year. As it is an environmentally safe technology that exploits natural power in an area that has not been fully explored yet, there is great potential in the future for expanding its use, especially in countries with narrow land mass or those that needs alternative renewable energy sources.
OSMOTIC POWER GENERATION PROTOTYPE:
Fresh water at sea level flows vertically downward through a penstock. The lower end of the penstock is situated about 90 meters below the sea surface where the pressure is 9 bars. This pressure forces a turbine to rotate and the pressure drops to 0 bar. Seawater is pumped from the surface to a barrier of semi permeable membranes (an osmotic unit). By osmosis the fresh water is driven through the membranes, trying to even out the amount of dissolved salt in the seawater. The flushing solution is pressurized to 9 bars and is pumped up to the surface. The diluted solution returns to the seawater by the osmotic pressure.
The osmotic effect is thus used to force the turbine to move. When the water is pressed out through the membranes due to a sucking effect, a stream appears. It is that stream, created by osmosis that makes the turbine spin. Thus, in neither of these plants osmosis is used for the direct generation of electric power. It is the sucking effect of the flow, which generates electric power
Improving the efficiency of output per square meter of membrane is the main challenge for the prototype plant the company explained.
NORWEGIAN COMPANY TO BUILD FIRST OSMOTIC POWER PLANT:
Norwegian energy company Statkraft has announced plans to harness usable energy from sea water by building the world’s first osmotic power plant. The company, which has invested £9m in developing the technology, it expects to have a commercially viable technology ready by 2015.
Statkraft estimates that globally osmotic power could generate 1,600TWh of power, including 200TWh in Norway accounting for 10 per cent of the country’s current energy use.
“They estimate they need five watts per square metre to make it commercially viable and are heading in the right direction.”
ENVIRONMENTAL PROSPECTS
The mixing of sea water and fresh water is a process that occurs in all river outlets in the world, and because rivers often run into the ocean in cities or other industrialized areas, most of the osmotic power potential can be utilized in urban areas. Osmotic power plants can be constructed partly or completely underground, thus allowing them to play a discrete role in the local environment
The water management processes associated with operation of the plant can be designed without affecting the biotopes of the river, river outlet and sea. In addition, osmotic power plant share very area efficient compared to other renewable energy sources.
DIFFERENT PLANTS USING OSMOSIS
SHEOPP Converter:
The picture below shows a SHEOPP Converter, which is a submarine hydroelectric power plant anchored to the sea floor. Fresh surface water, from a river mouth or an aqueduct, is conveyed through a penstock (standpipe) to a hydraulic turbine. After generating electric power, the fresh water is discharged and depressurized into a submarine tank. Finally the fresh water diffuses out in the sea by osmosis, through a barrier of semi permeable membranes.
For pure fresh water and perfect semi permeable membranes a flushing pump would not be necessary and the electric power produced in the SHEOPP Converter would be maximized. In real situations, however, the fresh water will generally contain non-tolerable amounts of dissolved salts and particles like sand, silt and other contaminants. It may then be necessary to pre treat the fresh water and a flushing pump would be required to prevent accumulation of unwanted solutes and contamination on the fresh water side of the membranes, to keep them in good working condition for as long as possible.
The efficiency for the SHEOPP will reach its maximum at a depth of 110 meters.
ABOUT PRO CONCEPT
The pressure retarded osmosis power plant is similar to a reverse osmosis desalination plant running backwards. However, the PRO plant generates power from freshwater instead of consuming power.
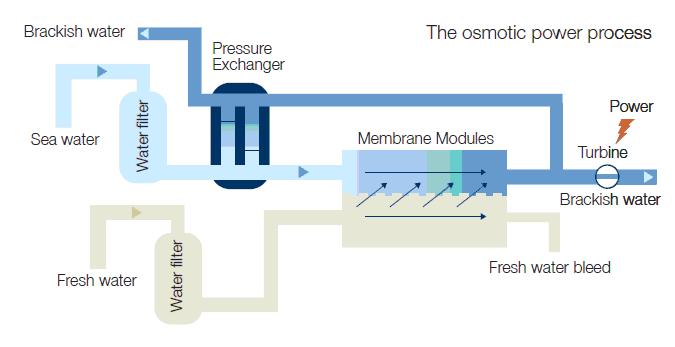
A simplified PRO process diagram is shown below.
Freshwater is fed into the plant and filtered before entering the membrane modules containing spiral wound or hollow fibre membranes. In the membrane module, 80 – 90 % of the fresh water is transferred by osmosis across the membrane into the pressurized
Seawater (bluish). The osmotic process increases the volumetric flow of high pressure water and is the key energy transfer in the plant.
This requires a membrane that has a high water flux and high salt retention. Typical membrane performance should be in the range of 4 – 6 W/m2.
The brackish water (dark blue) from the membrane module is split in two flows. About 1/3 of the water goes to the turbine to generate power. 2/3 return to the pressure exchanger to pressurize the feed of seawater. To optimize the power plant the typical operating pressure is in the range of 11 – 15 bars. This is equivalent to a water head of 100 – 145 meters in a hydropower plant, generating about 1 MW/ m3s freshwater. The freshwater feed operates at ambient pressure.
Some pre-treatment of the water is necessary. Experience from Norwegian water treatment plants shows that mechanical filtration down to 50 um in combination with a standard cleaning and maintenance cycle is enough to sustain the membrane performance for 7 – 10 years. Similar lifetime data are assumed for osmotic power PLANTS
CURRENT PROGESS
The PRO osmotic power process was invented in 1973 by Sidney Loeb, also known for his development of seawater reverse osmosis.
Some research was done in the 70’ies and 80’ies but no particular progress was made due to inefficient membranes.
Statkraft initiated PRO development in 1997. From 2001 Statkraft co-ordinated the world’s first major research project with the objective to develop osmotic power technology. Partly funded by the European Commission, the partners in the Salinity Power project were SINTEF (Norway), Forchungszentrum GKSS (Germany), Helsinki University of Technology (Finland) and ICTPOL (Portugal).
Membrane development Remarkable achievements have been made in improving the membrane performance Starting from less than 0.1 W/m2, the project verified power densities close to 2 W/m2. Experimental tests show that the best prototype membranes have properties equivalent to 5 W/m2, although this has to be verified in PRO operation.
FUTURE PLANS
Statkraft will maintain its world leading position in PRO and osmotic power production. In co-operation with strong industrial and scientific partners, Statkraft will continue its efforts to commercialize osmotic power. Improving the membrane performance to about 5 W/m2 in PRO operation and up-scaling the membrane units will beat focus over the next years.
Further plans will include the construction and verification of prototype systems. Later this will lead to full scale demonstration plants and introduction to the energy market
PROS AND CONS
There are many attractive features about using salt for power. A big advantage is that it is renewable. There is no risk what so ever to run out of salt because of osmotic produced power. (Salt-water evaporation leads to precipitation over land.) The process creating energy, does not consume the salt, it only utilizes it to force water to move. Another advantage is that osmotic-produced energy has a minimal environmental impact. It is a very “clean” process and this is of course a big plus. The amount of heat that occurs in the process would raise the temperature less than half a degree Celsius, which is not harmful to the marine organisms.
When we come to the disadvantages one big obstacle is the costs. Compared to other energy-producing processes osmotic energy is extremely expensive, about 36 times as expensive as a conventional power plant. There are also engineering problems to be overcome. It is difficult to build a large plant and lower it in the sea as deep as 110 meters, in the SHEOPP converter-case, and about 90 meters down in the ground, when it comes to the underground plant described earlier. Further there is a problem with the protection of the marine organisms from the turbine and other machinery.
FUTURE PROSPECTS
The possibility to use the salinity gradient in the ocean for power lies within the technology that needs to be developed. There are currently two hurdles to overcome, which includes the membrane water part and sunlight. If we could develop the membrane to use salt-water as fresh water and brine with a higher salt-concentration as the concentrated solution, then it would be more feasible to use salinity for power. However, the biggest hurdle that needs to be overcome is the cost.
CONCLUSION:
Osmotic power is a thriving renewable energy source Osmotic power plants can be constructed anywhere were freshwater flows out into the sea, provided that the salt concentration is sufficiently high. Unlike hydropower and wind power, osmotic power plants are not affected by fluctuations in the weather. The global potential is estimated to be an impressive 1600-1700 TWh .Researches are going on and prototypes of the plant have been built and the design is in further progress. However in the future if the technology is further developed and the costs will decrease, osmotic energy might be an alternative to the energy sources we use today.
REFERENCES: